DMT: Difference between revisions
| Line 504: | Line 504: | ||
<gallery mode="packed-hover"> | <gallery mode="packed-hover"> | ||
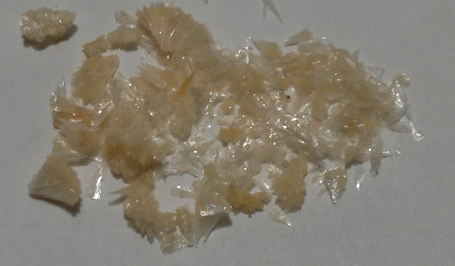
Image:Dmtwaxy. | Image:Dmtwaxy.png|''Waxy DMT'' | ||
Image:SpiceCrystal001.jpg|''Opaque DMT Crystal'' | Image:SpiceCrystal001.jpg|''Opaque DMT Crystal'' | ||
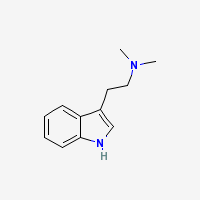
Image:DmtfreebaseMolecule.png|''DMT freebase molecule (N,N-DMT)'' | Image:DmtfreebaseMolecule.png|''DMT freebase molecule (N,N-DMT)'' | ||
Revision as of 18:56, 12 October 2014

General Information
N,N-Dimethyltryptamine or DMT for short is an short acting psychedelic entheogen which allows a person's consciousness to voyage into the most incredible dimensions, visions, thoughts and experiences imaginable. It is most commonly classified as a psychedelic but also possesses some properties inherent to dissociatives. It is one of the most powerful yet mysterious psychedelics in existence, but in the opinion of many users, to classify DMT as merely a drug would be doing it a great injustice as DMT seems to some as a transdimensional key into places and vistas so profound and awe inspiring that it raises many new questions regarding the nature of reality and our place within it. Nevertheless, it is important to realize that the experience may be very difficult for some to integrate, and great care and respect is necessary to use it.
DMT exists naturally in every human being and also throughout the plant and animal kingdoms. It occurs naturally in many mammals, marine animals, trees, grasses, flowers and shoots.
Plants Containing DMT
(from Block 1994*; Smith 1977; Montgomery, pers. comm.; Ott 1993*; Schultes and Hofman 1980, 155*; supplemented)
| Species | Demonstrated Tryptamines |
| AGARICACEAE (FuNGI) | |
| Amanita citrina Gray | DMT,5-MeO-DMT |
| Amanita porphyria (Fries) Secretan | 5-MeO-DMT |
| Amanita spp. | DMT, bufotenine |
| AIZOACEAE/MESEMBRYANTHEMACEAE | |
| Delosperma sp. | DMT,MMT |
| Mesembryanthemum spp. | DMT (?) |
| GRAMINEAE (POACEAE) | |
| Arundo donax 1. | DMT, bufotenine, and others |
| Phalaris arundinacea 1. | DMT, bufotenine, and others |
| Phalaris tuberosa 1. | DMT, bufotenine, and others |
| Phragmites australis (Cav.) Trin. ex Steud. | DMT |
| LAURACEAE | |
| Umbellularia californica (Hook. et A.) Nutt. | 5-MeO-DMT |
| LEGUMINOSAE | |
| Acacia confusa Merr. | DMT |
| Acacia maidenii F. von Mue1!. | DMT (0.360/0) |
| Acacia nubica Benth. | DMT |
| Acacia phlebophylla F. von Mue1!. | 0.30/0 DMT |
| Acacia simpIicifolia Druce | 0.810/0 DMT |
| Acaciaspp. | DMT |
| Anadenanthera colubrina (VeIl.) Bren. | DMT, 5-MeO-DMT, bufotenine |
| Anadenanthera peregrina (1.) Spag. | DMT, 5-MeO-DMT, bufotenine |
| Desmanthus illinoensis (Michx.) MacMillan | DMT (to 0.340/0) |
| Desmodium adscendens (Sw.) DC. var. adscendens | DMT (?) |
| Desmodium caudatum DC. | DMT |
| Desmodium gangeticum DC. | DMT, bufotenine, and others |
| Desmodium gyrans DC. | DMT, bufotenine, and others |
| Desmodium pulchellum Benth. ex Bak. | DMT, bufotenine, and others |
| Desmodium racemosum Thunb. | 5-MeO-DMT |
| Desmodium tiliaefolium G. Don | DMT, bufotenine, and others |
| Desmodium triflorum DC. | DMT, bufotenine, and others |
| Lespedeza bicolor Turcz. | DMT, 5-MeO-DMT |
| Lespedeza bicolor var. japonica Nakai | DMT,5-MeO-DMT |
| Lespedeza capitata Michx. | DMT |
| Mimosa scabrella Benth. | DMT |
| Mimosa tenuiflora (Willd.) Pair. [syn. Mimosa hostilis | 0.570/0 DMT |
| Benth., Mimosa nigra] | |
| Mimosa verrucosa | DMT |
| Mimosaspp. | DMT and others |
| Mucuna pruriens DC. | DMT, 5-MeO-DMT, bufotenine |
| Mucunaspp. | DMT and others |
| Petalostylis cassioides Pritze1 | DMT, tetrahydroharmane |
| Petalostylis labicheoides R. Brown | DMT, tryptamine |
| Phyllodium pulchellum (1.) Desv. | DMT |
| MALPIGHIACEAE | |
| Banisteriopsis argentea Spring. [syn. B. muricata (Cav.) Cuatr.] | DMT, DMT |
| Diplopterys cabrerana (Cuatr.) Gates | DMT,5 |
| [syn. Banisteriopsis rusbyana] | |
| MYRISTICACEAE | |
| Iryanthera ulei Warb. | 5-MeO-DMT |
| Osteophloeum platyspermum (DC.) Warb. | DMT,5-MeO-DMT |
| Viroia calophylla Warb. | DMT,5-MeO-DMT |
| Virola calophylloidea Markgr. | DMT,5-MeO-DMT |
| Virola carinata (Spruce ex Benth.) Warb. | DMT,5-MeO-DMT |
| Virola divergens Ducke | DMT |
| Virola elongata (Spruce ex Benth.) Warb. | DMT, 5-MeO-DMT |
| Virola melinonii (Benoist) A.C. Smith | DMT,5-MeO-DMT |
| Virola multinerva Ducke | DMT,5-MeO-DMT |
| Virola pavonis (DC.) A.C. Smith | DMT |
| Virola peruviana (DC.) Warb. | DMT,5-MeO-DMT |
| Virola rufula (DC.) Warb. | DMT,5-MeO-DMT |
| Virola sebifera Aubi. | DMT |
| Virola theiodora (Spruce ex Benth.) Warb. | DMT,5-MeO-DMT |
| Virola venosa (Benth.) Warb. | DMT, 5-MeO-DMT, and others |
| Virolaspp. | |
| OCHNACEAE | |
| Testulea gabonensis Pellegr. | DMT |
| POLYGONACEAE | |
| Eriogonum sp. | DMT |
| RUBIACEAE | |
| Psychotria carthaginensis Jacq. | DMT |
| Psychotria poeppigiana Mueli. Arg. | DMT |
| Psychotria viridis Ruiz et Pay. [syn. P. psychotriaefolia Stand!.] | DMT |
| RUTACEAE | |
| Dictyoloma incanescens DC. | 5-MeO-DMT |
| Dutaillyea drupacea (Baill.) Hartley | 5-MeO-DMT |
| Dutaillyea oreophila (Baill.) Sevenet-Pusset | 5-MeO-DMT |
| Evodia rutaecarpa Benth. | 5-MeO-DMT |
| Limonia acidissima 1. | DMT traces |
| Melicope leptococca (Baill.) Guill. | 0.21% DMT |
| Pilocarpus organensis Rizzini et Occhioni | 5-MeO-DMT |
| Vepris ampody H. Perro | DMT |
| Zanthoxylum arborescens Rose | DMT traces |
| Zanthoxylum procerum Donn. Sm. | DMT |
Dosage
Oral
DMT is rapidly metabolized by MAO and should therefore be combined with a MAO-inhibitor such as Harmine when taken orally. Otherwise, the effects will be underwhelming or barely noticeable.
Dosages for DMT, considering MAOs are fully inhibited, vary wildly depending on person, probably due to metabolism in great part. They can go from 30 to 150mg! If its your first time, start on the lower end!
Another factor is whether one is ingesting a whole plant brew or purified extracts. Often in ayahuasca analysis the amount of DMT found is very small (20-30mg), but also often there is redosing in ayahuasca sessions, but also its possible trace amounts of beta-carbolines and other alkaloids can improve MAO inhibition, or that other inactive plant substances can help protect DMT from fast breakdown by any potential MAO activity.
There are a few different ways to ingest it orally:
- Dissolved in acidic juice.
- Rolled inside a bit of smoking paper and swallowed like a pill.
- Put into 00 Capsules.
| Light | 30-50mg of DMT & 50-75mg Harmala alkaloids |
| Common | 50-75mg of DMT & 75-100mg Harmala alkaloids |
| Strong | 100mg+ of DMT & 100-150mg Harmala alkaloids |
Smoked-Vaporized
Dosage given assumes 100% effective vaporization method.
| Light | 10-15mg |
| Common | 15-25mg |
| Strong | 25-40mg+ |
Extracted DMT freebase can be vaporized for very potent effects that last around 10-15 minutes. DMT is ideally vaporized, as opposed to smoked. Vaporization is achieved by a controlled temperature that does not burn/combust DMT material (and potential impurities), but instead just makes DMT evaporate and be inhaled.
Vaporization is much smoother than smoking. Smoking leads to break down of DMT (and impurity) molecules into potential toxic nitrogen oxides, so not only it is harsher but also there is a significant loss of actives.
Vaporizing can be achieved with improvised vaporizers such as "The Inspirator mk II","The Machine" (both DIY) or commercially sold vaporizing pipes such as the Vapor Genie.
Insufflated
Insufflated doses vary wildly, start low. Dosages given are for freebase DMT, dosage for salt form may be a little bit higher.
| Light | 10-25mg |
| Common | 25-50mg |
| Strong | 50-125mg+ |
Insufflation of DMT is a less popular method of administration because it can cause extreme physical discomfort. DMT can be snorted in both freebase and salt forms. The most common salt utilized for insufflation is DMT Fumarate. Note that highly basic substances can damage the nasal passageway so the potential user is advised to proceed with caution. Preparations to make DMT insuflation more tolerable from DMT-Nexus
Intravenous
Intravenous DMT comes on extremely fast and is the most efficient method of use. The effects are extremely similar to vaporized DMT, though IV administration is much more dangerous. It is advised that potential users avoid intravenous administration of DMT and stick to vaporization.
| Light | 10-15mg |
| Common | 15-25mg |
| Strong | 25-40mg+ |
Duration
| Onset | 10-60 minutes |
| Total | 4-8 hours |
| After-effects | 2-3 hours |
| Onset | 0-3 minutes |
| Peak | 10-15 minutes |
| Total | 15-30 minutes |
| Onset | 1-5 minutes |
| Total | 45-60 minutes |
| Onset | >1 minute |
| Total | 30-45 minutes |
Effects
Positive
- Euphoria
- Feeling of awe
- Realizations about one's life
Neutral
- Time dilation
- Intense hallucinations
- Dissociation
- Loss of comprehension of basic concepts such as ego, language or one's own body.
- Out-of-body experiences
- Unconventional thought patterns
Negative
- Panic attack/Bad trip
- (oral only) Nausea/Vomitting
After effects
- A lasting perspective shift is likely to occur.
- Depersonalization/Derealization can occur after an experience and may last up to several months.
- Some people experience long-term anxiety after an experience while others report a decrease in overall anxiety.
Harm Reduction
If ingesting DMT orally with a MAOI, ensure that care is taken with the diet such that no food which may cause an interaction is ingested - as this is potentially deadly. Check Erowid's MAOI Foods To Avoid page for more information. However, if using a reversible MAOI such as Syrian Rue, less care needs to be taken.
Refer to Psychedelic Harm Reduction for general information on psychedelic safety.
Images
-
Waxy DMT
-
Opaque DMT Crystal
-
DMT freebase molecule (N,N-DMT)
-
Changa
Chemistry and Pharmacology
DMT is closely related to serotonin, the naturally occurring neurotransmitter that psychedelics affect so widely. The pharmacology of DMT is similar to that of other well-known psychedelics. It affects receptor sites for serotonin in much the same way that LSD, psilocybin, and mescaline do. These serotonin receptors are widespread throughout the body and can be found in blood vessels, tissues, muscle, glands, and skin.
Production
There are a number of ways to acquire this entheogen. The first and most difficult way is to have some substantial chemistry knowledge and experience and actually synthesize pure DMT in a laboratory. This a rather tricky and time consuming process and requires access to some rather obscure and hard to acquire chemicals. The most common and easiest method to acquire DMT is to extract it from the various plant species that contain the compound. DMT occurs naturally in several plants and may therefore be extracted from them with little chemistry knowledge, requiring no actual synthesis.
Plants containing DMT include Mimosa hostilis/tenuflora, Psychotria viridis, Codariocalyx motorius, Diplopterys cabrerana, Acacia species, and Phalaris species.
Extraction Teks
Legal status
Europe
DMT is a class A substance in most European countries.
America
DMT is a schedule I substance in the United States.