2C-B: Difference between revisions
GrimReaper (talk | contribs) |
|||
| Line 1: | Line 1: | ||
[[File:2C-B-Chemdraw.png|200px|right|Chemical structure of 2C-B]] | [[File:2C-B-Chemdraw.png|200px|right|Chemical structure of 2C-B]] | ||
'''2C-B''' is a psychedelic drug of the [[2C-X|2C-X family]]. | |||
Effects are often described as being more easily managed than other psychedelics; it is often compared to a mixture of a LSD and MDMA. | |||
2C-B is also known for the strong body component of its effects which are alternately described as pleasurable energy or a 'sense of being in the body', and by others as an unpleasant 'buzzing' or body-load, which is mostly occurring during onset. | |||
= History = | = History = | ||
2C-B was first synthesized in 1974 by Alexander Shulgin. It was first sold commercially as an aphrodisiac under the trade name "Eros" by the German pharmaceutical company | 2C-B was first synthesized in 1974 by Alexander Shulgin. It first saw use among the psychiatric community as an aid during therapy. It was considered one of the best drugs for this purpose because of its short duration, relative absence of side effects, and comparably mild nature.[2] Shortly after becoming popular in the medical community, it became popular recreationally. 2C-B was first sold commercially as an aphrodisiac under the trade name "Eros", which was manufactured by the German pharmaceutical company Drittewelle. From many years after it was available as tablets in Dutch smart shops under the name "Nexus". | ||
= Dosage = | = Dosage = | ||
| Line 52: | Line 54: | ||
= Effects = | = Effects = | ||
== Positive == | == Positive == | ||
| Line 96: | Line 97: | ||
* Nexus | * Nexus | ||
= Chemistry and Pharmacology = | |||
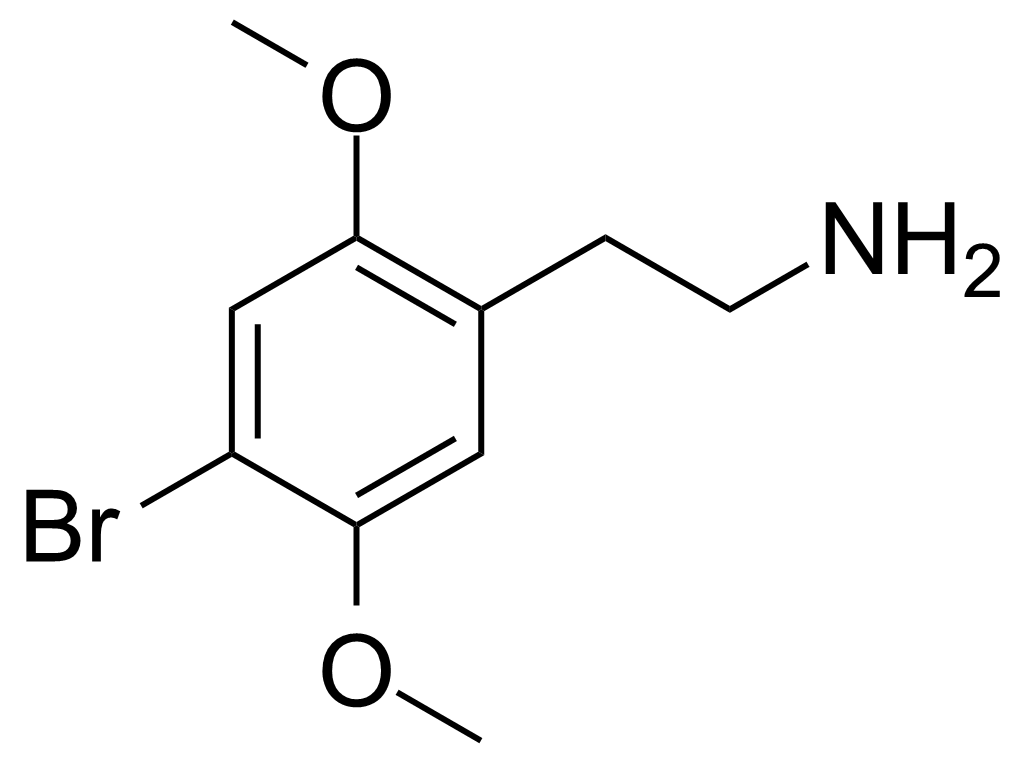
Systematic name: 2-(4-bromo-2,5-dimethoxyphenyl)ethanamine | |||
Unlike most hallucinogens, 2C-B has been shown to be a low efficacy serotonin 5-HT2A receptor partial agonist or even full antagonist. This suggests that the 5-HT2C receptor is primarily responsible for mediating the effects experienced by users of 2C-B, although functional antagonism of 5-HT2A or activation of the 5-HT2A-coupled phospholipase D pathway may also play a role. The rank order of receptorantagonist potency for this family of drugs is 2C-I > 2C-B > 2C-D > 2C-H. | |||
Research suggests that 2C-B increases dopamine levels in the brains of rats, which may contribute to its psychoactivity. | |||
= Harm Reduction = | = Harm Reduction = | ||
| Line 102: | Line 109: | ||
= Legal Status = | = Legal Status = | ||
Internationally, 2C-B is a Schedule II drug under the Convention on Psychotropic Substances. In the Netherlands, 2C-B became a list I substance of the Opium Law despite no health incidents occurring. Following the ban, other phenethylamines were sold in place of 2C-B until the Netherlands became the first country in the world to ban 2C-I, 2C-T-2 and 2C-T-7 alongside 2C-B. | |||
* Europe: Class A (Along with all the others in the [[2C-X|2C-X family]]. (Illegal to produce, supply, or possess.)) | * Europe: Class A (Along with all the others in the [[2C-X|2C-X family]]. (Illegal to produce, supply, or possess.)) | ||
Revision as of 06:56, 16 July 2014
2C-B is a psychedelic drug of the 2C-X family. Effects are often described as being more easily managed than other psychedelics; it is often compared to a mixture of a LSD and MDMA. 2C-B is also known for the strong body component of its effects which are alternately described as pleasurable energy or a 'sense of being in the body', and by others as an unpleasant 'buzzing' or body-load, which is mostly occurring during onset.
History
2C-B was first synthesized in 1974 by Alexander Shulgin. It first saw use among the psychiatric community as an aid during therapy. It was considered one of the best drugs for this purpose because of its short duration, relative absence of side effects, and comparably mild nature.[2] Shortly after becoming popular in the medical community, it became popular recreationally. 2C-B was first sold commercially as an aphrodisiac under the trade name "Eros", which was manufactured by the German pharmaceutical company Drittewelle. From many years after it was available as tablets in Dutch smart shops under the name "Nexus".
Dosage
NOTE: DO NOT TAKE THE BELOW DOSING INFORMATION AS A KNOW ALL, PLEASE TAKE CAUTION, AND REMEMBER THAT YOU CAN ALWAYS TAKE MORE, BUT NEVER LESS.
Oral
Light: 5-15 mg
Common: 15-30 mg
Strong: 30-50 mg
Heavy: 50+ mg
Insufflated
Light: 5-10 mg
Common: 10-20 mg
Strong: 20-30 mg
Heavy: 30+ mg
Rectal
Light: 5-10 mg
Common: 10-20 mg
Strong: 20-30 mg
Heavy: 30+ mg
Duration
Oral
Onset: 20-75 minutes
Total duration: 4-8 hours
Insufflated
Onset: 1-10 minutes
Total duration: 4-8 hours
Rectal
Onset: 5-20 minuted
Total duration: 4-8 hours
Effects
Positive
- Euphoria
- Giggling
- Empathy
- Personal Insight
- Enhanced Colours
- Closed and Open Eye Visuals
- Enhanced Tactile Sensation
Neutral
- Decreased Appetite
- Pupil Dilation
- Time Dilation
Negative
- Restlessness
- Sweating/Chills
- Nausea
- Insomnia
- Muscle Tension
- Confusion
Aliases
- Bees
- Nexus
Chemistry and Pharmacology
Systematic name: 2-(4-bromo-2,5-dimethoxyphenyl)ethanamine Unlike most hallucinogens, 2C-B has been shown to be a low efficacy serotonin 5-HT2A receptor partial agonist or even full antagonist. This suggests that the 5-HT2C receptor is primarily responsible for mediating the effects experienced by users of 2C-B, although functional antagonism of 5-HT2A or activation of the 5-HT2A-coupled phospholipase D pathway may also play a role. The rank order of receptorantagonist potency for this family of drugs is 2C-I > 2C-B > 2C-D > 2C-H.
Research suggests that 2C-B increases dopamine levels in the brains of rats, which may contribute to its psychoactivity.
Harm Reduction
2C-B is known as one of the safer psychedelics, with several reported cases of users far exceeding commonly used dosing limits without lasting adverse physical effects. It is also observed to have a low addiction potential. It is a stimulating psychedelic, and therefore it's important to remain hydrated. Refer to Psychedelic Harm Reduction for more information.
Legal Status
Internationally, 2C-B is a Schedule II drug under the Convention on Psychotropic Substances. In the Netherlands, 2C-B became a list I substance of the Opium Law despite no health incidents occurring. Following the ban, other phenethylamines were sold in place of 2C-B until the Netherlands became the first country in the world to ban 2C-I, 2C-T-2 and 2C-T-7 alongside 2C-B.
- Europe: Class A (Along with all the others in the 2C-X family. (Illegal to produce, supply, or possess.))
- United States: Schedule I (Illegal to produce, supply, or possess.)