Hydromorphone: Difference between revisions
Jump to navigation
Jump to search
| Line 14: | Line 14: | ||
== Dosage == | == Dosage == | ||
== Effects == | |||
=== Positive === | |||
* Euphoria | |||
* Pain relief | |||
=== Neutral === | |||
* Pupil Constriction | |||
* Itching | |||
* Sedation | |||
=== Negative === | |||
* Drowsiness | |||
* CNS depression | |||
* Dizziness | |||
* Nausea | |||
* Vomiting | |||
* Constipation | |||
* Sweating | |||
== Legal Status == | == Legal Status == | ||
* United States: [http://www.justice.gov/dea/druginfo/ds.shtml Schedule II] | * United States: [http://www.justice.gov/dea/druginfo/ds.shtml Schedule II] | ||
Revision as of 07:31, 12 December 2013
General information
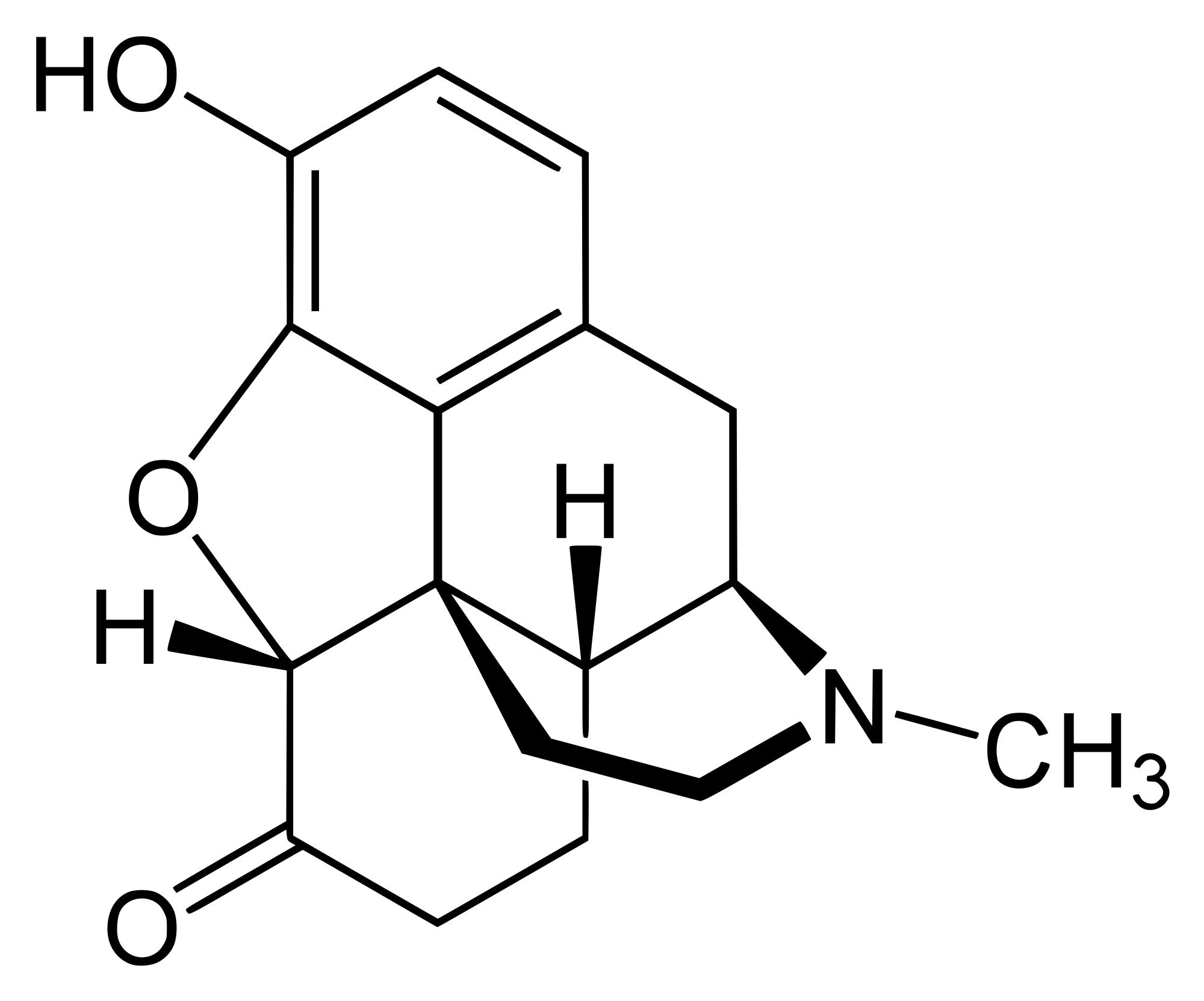
Hydromorphone, usually sold as brand names Palladone or Dilaudid, is a very potent centrally acting analgesic drug of the opioid class.
History
Knoll introduced it to the mass market in 1926 under the brand name Dilaudid, indicating its derivation and degree of similarity to morphine (by way of laudanum). The brand name Dilaudid is more widely known than the generic term hydromorphone, and because of this, Dilaudid is often used generically to mean any form of hydromorphone.
Images
Dosage
Effects
Positive
- Euphoria
- Pain relief
Neutral
- Pupil Constriction
- Itching
- Sedation
Negative
- Drowsiness
- CNS depression
- Dizziness
- Nausea
- Vomiting
- Constipation
- Sweating
Legal Status
- United States: Schedule II