Benzodiazepines: Difference between revisions
m |
|||
| (32 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:Xanax.jpg|thumb|250px|Alprazolam pills and prescription bottle]] | |||
Benzodiazepines are drugs which act upon the GABA(A) receptor, and produce a general set of effects which vary by compound, mostly being sedative, hypnotic, anxiolytic, anticonvulsant and muscle relaxant. The first benzodiazepine, Chlordiazepoxide (Librium), was discovered accidentally by Leo Sternbach in 1955, and made available in 1960 by Hoffmann–La Roche, which has also marketed diazepam (Valium) since 1963. | Benzodiazepines are drugs which act upon the GABA(A) receptor, and produce a general set of effects which vary by compound, mostly being sedative, hypnotic, anxiolytic, anticonvulsant and muscle relaxant. The first benzodiazepine, Chlordiazepoxide (Librium), was discovered accidentally by Leo Sternbach in 1955, and made available in 1960 by Hoffmann–La Roche, which has also marketed diazepam (Valium) since 1963. | ||
| Line 10: | Line 13: | ||
! Class | ! Class | ||
|- | |- | ||
| Alprazolam (Xanax) | | [[Alprazolam|Alprazolam (Xanax)]] | ||
| 6 - 12 hours | | 6 - 12 hours | ||
| 0.5 mg | | 0.5 mg | ||
| Line 20: | Line 23: | ||
| Anxiolytic | | Anxiolytic | ||
|- | |- | ||
| Brotizolam | | Brotizolam (Lendormin) | ||
| 2 - 6 hours | | 2 - 6 hours | ||
| .25mg | | .25mg | ||
| Hypnotic | | Hypnotic | ||
|- | |- | ||
| | | Chlordiazepoxide (Librium) | ||
| 5 - 30 hours [36 - 200 hours] | | 5 - 30 hours [36 - 200 hours] | ||
| 25 mg | | 25 mg | ||
| Anxiolytic | | Anxiolytic | ||
|- | |- | ||
| Clobazam (Frisium) | | Clobazam (Frisium, Urbanol) | ||
| 12 - 60 hours | | 12 - 60 hours | ||
| 20 mg | | 20 mg | ||
| Line 38: | Line 41: | ||
| 18 - 50 hours | | 18 - 50 hours | ||
| 0.5 mg | | 0.5 mg | ||
| Anxiolytic | |||
|- | |||
| [[Clonazolam]] | |||
| N/A | |||
| 0.2 mg | |||
| Anxiolytic | | Anxiolytic | ||
|- | |- | ||
| Line 45: | Line 53: | ||
| Anxiolytic | | Anxiolytic | ||
|- | |- | ||
| Diazepam (Valium) | | [[Diazepam|Diazepam (Valium)]] | ||
| 20 - 100 hours [36 - 200 hours] | | 20 - 100 hours [36 - 200 hours] | ||
| 10 mg | | 10 mg | ||
| Anxiolytic | | Anxiolytic | ||
|- | |- | ||
| Diclazepam | | Diclazepam (Ro5-3448) | ||
| ~ | | ~42 hours | ||
| 1mg | | 1mg | ||
| Anxiolytic | | Anxiolytic | ||
| Line 60: | Line 68: | ||
| Hypnotic | | Hypnotic | ||
|- | |- | ||
| Etizolam | | [[Etizolam|Etizolam (Etilaam, Etizola, Etizest, Depas)]] | ||
| 4-12 hours | | 4-12 hours | ||
| 1mg | | 1mg | ||
| Line 70: | Line 78: | ||
| Hypnotic | | Hypnotic | ||
|- | |- | ||
| Flunitrazepam (Rohypnol) | | Flubromazolam | ||
| Long | |||
| .25mg | |||
| Hypnotic | |||
|- | |||
| [[Flunitrazepam|Flunitrazepam (Rohypnol)]] | |||
| 18 - 26 hours [36 - 200 hours] | | 18 - 26 hours [36 - 200 hours] | ||
| 1 mg | | 1 mg | ||
| Line 109: | Line 122: | ||
| 1 - 2 mg | | 1 - 2 mg | ||
| Hypnotic | | Hypnotic | ||
|- | |||
| Midazolam (Dormicum) | |||
| 1.6 - 8.3 hours | |||
| 10mg | |||
| Hypnotic | |||
|- | |- | ||
| Medazepam (Nobrium) | | Medazepam (Nobrium) | ||
| Line 161: | Line 179: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable sortable" | ||
|+Non-benzodiazepines commonly referred to as Z-drugs | |+Non-benzodiazepines commonly referred to as Z-drugs | ||
! Chemical name (brand name) | ! Chemical name (brand name) | ||
| Line 173: | Line 191: | ||
| Hypnotic | | Hypnotic | ||
|- | |- | ||
| Zolpidem (Ambien) | | [[Zolpidem|Zolpidem (Ambien)]] | ||
| 2 hours | | 2 hours | ||
| 20 mg | | 20 mg | ||
| Line 199: | Line 217: | ||
* High addiction potential | * High addiction potential | ||
* | * Withdrawals can be fatal | ||
* Risk of blackout | * Risk of blackout | ||
* Inability to drink | * Inability to drink | ||
* Inability to | * Inability to drive | ||
* Loss of balance | * Loss of balance | ||
* Memory Loss | * Memory Loss | ||
* Procrastination | * Procrastination | ||
* "Hangover" | * "Hangover" | ||
* Long term effects | * Long-term effects | ||
* High addiction potential | * High addiction potential | ||
| Line 228: | Line 246: | ||
* CNS depressant when mixed with other drugs | * CNS depressant when mixed with other drugs | ||
= | == Research Chemicals == | ||
The main danger of the drugs in this class is the risk of blacking out or overdosing by mixing it with other CNS depressants. | |||
Most of the research chemicals from this class are usually active under 10mg's. The best course of action with these substances is putting X amount of active chemical into X amount of your solvent of choice (Such as Propylene-Glycol or Ethanol) And using an oral syringe to get the dose you want. Granted a fair amount are sold are either in presses or on a blotter. | |||
See the [[Research Chemicals]] page for more information. | |||
= Chemistry and Pharmacology = | = Chemistry and Pharmacology = | ||
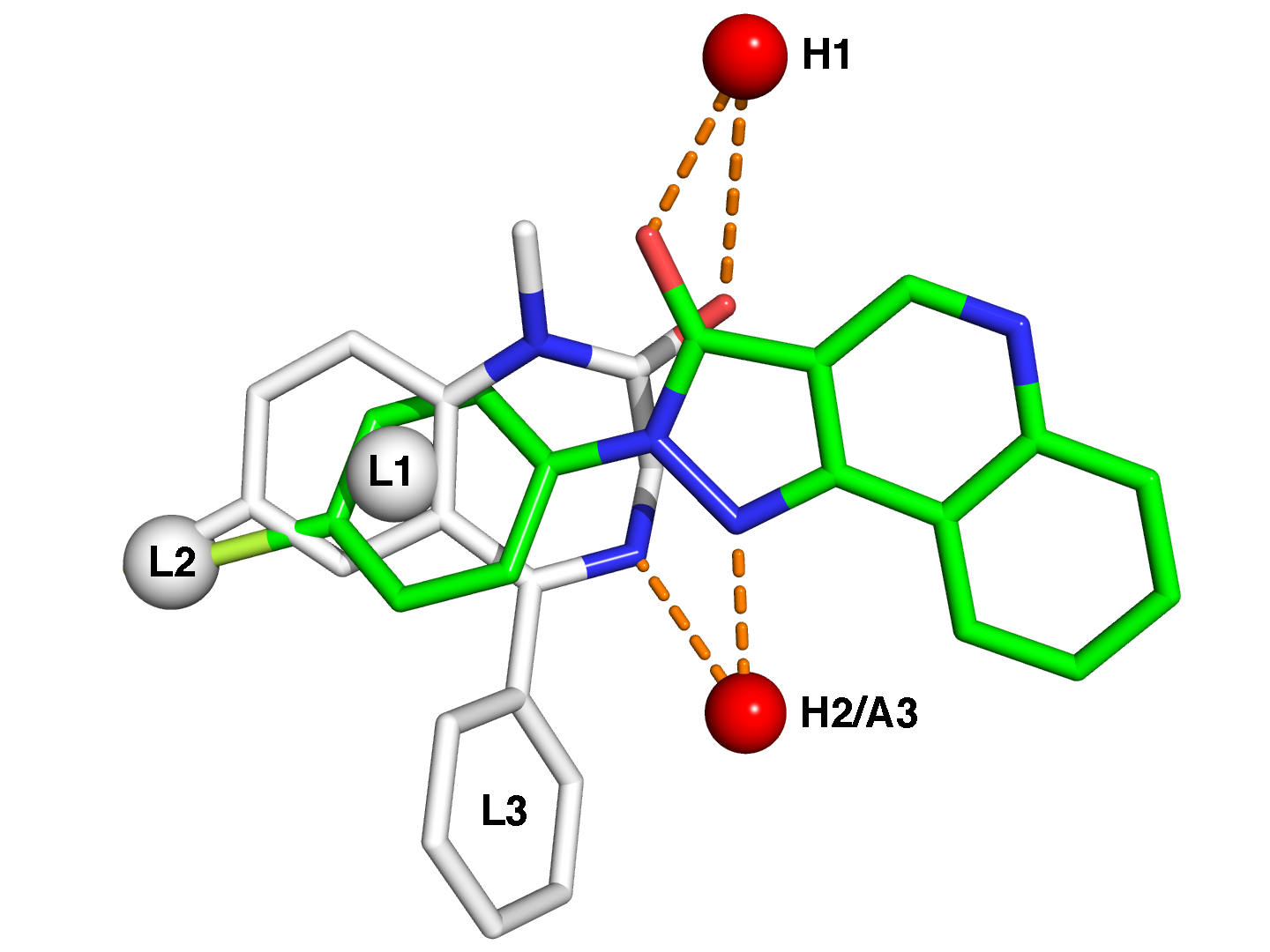
The term benzodiazepine is the chemical name for the heterocyclic ring system, which is a fusion between the benzene and diazepine ring systems. Under Hantzsch–Widman nomenclature, a diazepine is a heterocycle with two nitrogen atoms, five carbon atom and the maximum possible number of cumulative double bonds. The "benzo" prefix indicates the benzene ring fused onto the diazepine ring. | The term benzodiazepine is the chemical name for the heterocyclic ring system, which is a fusion between the benzene and diazepine ring systems. Under Hantzsch–Widman nomenclature, a diazepine is a heterocycle with two nitrogen atoms, five carbon atom and the maximum possible number of cumulative double bonds. The "benzo" prefix indicates the benzene ring fused onto the diazepine ring. | ||
Benzodiazepine drugs are substituted 1,4-benzodiazepines, although the chemical term can refer to many other compounds that do not have useful pharmacological properties. Different benzodiazepine drugs have different side groups attached to this central structure. The different side groups affect the binding of the molecule to the GABAA receptor and so modulate the pharmacological properties. Many of the pharmacologically active "classical" benzodiazepine drugs contain the 5-phenyl-1H-benzo[e][1,4]diazepin-2(3H)-one substructure. | Benzodiazepine drugs are substituted 1,4-benzodiazepines, although the chemical term can refer to many other compounds that do not have useful pharmacological properties. Different benzodiazepine drugs have different side groups attached to this central structure. The different side groups affect the binding of the molecule to the GABAA receptor and so modulate the pharmacological properties. Many of the pharmacologically active "classical" benzodiazepine drugs contain the 5-phenyl-1H-benzo[e][1,4]diazepin-2(3H)-one substructure. | ||
Nonbenzodiazepines also bind to the benzodiazepine binding site on the GABA(A) receptor and possess similar pharmacological properties. While the nonbenzodiazepines are by definition structurally unrelated to the benzodiazepines, both classes of drugs possess a common pharmacophore , which explains their binding to a common receptor site | Nonbenzodiazepines also bind to the benzodiazepine binding site on the GABA(A) receptor and possess similar pharmacological properties. While the nonbenzodiazepines are by definition structurally unrelated to the benzodiazepines, both classes of drugs possess a common pharmacophore, which explains their binding to a common receptor site | ||
[[File:benzo.png|500px]] | [[File:benzo.png|500px]] | ||
| Line 251: | Line 269: | ||
::Clonazepam, Flunitrazepam, Nimetazepam, Nitrazepam | ::Clonazepam, Flunitrazepam, Nimetazepam, Nitrazepam | ||
* Triazolo compounds: | * Triazolo compounds: | ||
::Adinazolam, Alprazolam, Estazolam, Triazolam | ::Adinazolam, Alprazolam, Clonazolam, Estazolam, Triazolam | ||
* Imidazo compounds | * Imidazo compounds | ||
::Climazolam, Loprazolam, Midazolam | ::Climazolam, Loprazolam, Midazolam | ||
- | = Images = | ||
<gallery mode="packed-hover" heights="200px"> | |||
Image:Xanax.jpg|''(Purepac) alprazolam pills and prescription bottle'' | |||
Image:Alprazolam_solution.jpg|''Alprazolam solved in propylene glycol'' | |||
Image:Alpraz.jpg|''.5mg (Qualitest) alprazolam pills'' | |||
Image:Ativan.png|''Lorazepam (Ativan) pill'' | |||
</gallery> | |||
= Links = | = Links = | ||
| Line 273: | Line 298: | ||
Carlo, Pia; Renata Finollo, Anna Ledda, Giovanni Brambilla (January 1989). "Absence of liver DNA fragmentation in rats treated with high oral doses of 32 benzodiazepine drugs". Fundamental and Applied Toxicology 12 (1): 34–41. doi:10.1016/0272-0590(89)90059-6. PMID 2925017. | Carlo, Pia; Renata Finollo, Anna Ledda, Giovanni Brambilla (January 1989). "Absence of liver DNA fragmentation in rats treated with high oral doses of 32 benzodiazepine drugs". Fundamental and Applied Toxicology 12 (1): 34–41. doi:10.1016/0272-0590(89)90059-6. PMID 2925017. | ||
[[Category: | [[Category:Drug class]] | ||
Latest revision as of 18:15, 7 July 2018
Benzodiazepines are drugs which act upon the GABA(A) receptor, and produce a general set of effects which vary by compound, mostly being sedative, hypnotic, anxiolytic, anticonvulsant and muscle relaxant. The first benzodiazepine, Chlordiazepoxide (Librium), was discovered accidentally by Leo Sternbach in 1955, and made available in 1960 by Hoffmann–La Roche, which has also marketed diazepam (Valium) since 1963.
Dosage
| Chemical name (brand name) | Half-Life [Active Metabolites] | Dose Equiv. of 10mg Diazepam (Oral) | Class |
|---|---|---|---|
| Alprazolam (Xanax) | 6 - 12 hours | 0.5 mg | Anxiolytic |
| Bromazepam (Lexotan, Lexomil) | 10 - 20 hours | 5 - 6 mg | Anxiolytic |
| Brotizolam (Lendormin) | 2 - 6 hours | .25mg | Hypnotic |
| Chlordiazepoxide (Librium) | 5 - 30 hours [36 - 200 hours] | 25 mg | Anxiolytic |
| Clobazam (Frisium, Urbanol) | 12 - 60 hours | 20 mg | Anxiolytic |
| Clonazepam (Klonopin) | 18 - 50 hours | 0.5 mg | Anxiolytic |
| Clonazolam | N/A | 0.2 mg | Anxiolytic |
| Clorazepate (Tranxene) | [36 - 200 hours] | 15 mg | Anxiolytic |
| Diazepam (Valium) | 20 - 100 hours [36 - 200 hours] | 10 mg | Anxiolytic |
| Diclazepam (Ro5-3448) | ~42 hours | 1mg | Anxiolytic |
| Estazolam (ProSom, Nuctalon) | 10 - 24 hours | 1 - 2 mg | Hypnotic |
| Etizolam (Etilaam, Etizola, Etizest, Depas) | 4-12 hours | 1mg | Anxiolytic |
| Flubromazepam | 106 hours | 6 - 8 mg | Hypnotic |
| Flubromazolam | Long | .25mg | Hypnotic |
| Flunitrazepam (Rohypnol) | 18 - 26 hours [36 - 200 hours] | 1 mg | Hypnotic |
| Flutoprazepam (Restas) | 60 - 90 hours | ~2.5 mg | Hypnotic |
| Flurazepam (Dalmane) | [40 - 250 hours] | 15 - 30 mg | Hypnotic |
| Halazepam (Paxipam) | [30 - 100 hours] | 20 mg | Anxiolytic |
| Ketazolam (Anseren) | 30 - 100 hours [36 - 200 hours] | 15 - 30 mg | Anxiolytic |
| Loprazolam (Dormonoct) | 6 - 12 hours | 1 - 2 mg | Hypnotic |
| Lorazepam (Ativan) | 10 - 20 hours | 1 mg | Anxiolytic |
| Lormetazepam (Noctamid) | 10 - 12 hours | 1 - 2 mg | Hypnotic |
| Midazolam (Dormicum) | 1.6 - 8.3 hours | 10mg | Hypnotic |
| Medazepam (Nobrium) | 36 - 200 hours | 10 mg | Anxiolytic |
| Nitrazepam (Mogadon) | 15 - 38 hours | 10 mg | Hypnotic |
| Nordazepam (Nordaz) | 36 - 200 hours | 10 mg | Anxiolytic |
| Oxazepam (Serax) | 4 - 15 hours | 20 mg | Anxiolytic |
| Phenazepam | 60 hours | ~1 mg. | Hypnotic |
| Prazepam (Centrax) | [36 - 200 hours] | 10 - 20 mg | Anxiolytic |
| Pyrazolam | Short | .83 mg | Anxiolytic |
| Quazepam (Doral) | 25 - 100 hours | 20 mg | Hypnotic |
| Temazepam (Restoril) | 8 - 22 hours | 20 mg | Hypnotic |
| Triazolam (Halcion) | 2 hours | 0.5 mg | Hypnotic |
| Chemical name (brand name) | Half-Life [Active Metabolites] | Dose Equiv. of 10mg Diazepam (Oral) | Class |
|---|---|---|---|
| Zaleplon (Sonata) | 2 hours | 20 mg | Hypnotic |
| Zolpidem (Ambien) | 2 hours | 20 mg | Hypnotic |
| Zopiclone (Imovane) | 5 - 6 hours | 15 mg | Hypnotic |
| Eszopiclone (Lunesta) | 6 hours | 3 mg | Hypnotic |
Effects
Positive
- Anti-Anxiety
- Sedative
- Muscle relaxant
Negative
- High addiction potential
- Withdrawals can be fatal
- Risk of blackout
- Inability to drink
- Inability to drive
- Loss of balance
- Memory Loss
- Procrastination
- "Hangover"
- Long-term effects
- High addiction potential
Harm Reduction
- Avoid driving and operating machinery
- Recommended time (pauses) between using the substance
- Addiction potential - High
- Risk of blackouts
- Risk of death when mixed with alcohol or other drugs. An extremely high percentage of drug-related deaths are due to mixing benzos with other drugs, especially opiates and alcohol.
- Mental illness
- Heart issues
- CNS depressant when mixed with other drugs
Research Chemicals
The main danger of the drugs in this class is the risk of blacking out or overdosing by mixing it with other CNS depressants.
Most of the research chemicals from this class are usually active under 10mg's. The best course of action with these substances is putting X amount of active chemical into X amount of your solvent of choice (Such as Propylene-Glycol or Ethanol) And using an oral syringe to get the dose you want. Granted a fair amount are sold are either in presses or on a blotter.
See the Research Chemicals page for more information.
Chemistry and Pharmacology
The term benzodiazepine is the chemical name for the heterocyclic ring system, which is a fusion between the benzene and diazepine ring systems. Under Hantzsch–Widman nomenclature, a diazepine is a heterocycle with two nitrogen atoms, five carbon atom and the maximum possible number of cumulative double bonds. The "benzo" prefix indicates the benzene ring fused onto the diazepine ring. Benzodiazepine drugs are substituted 1,4-benzodiazepines, although the chemical term can refer to many other compounds that do not have useful pharmacological properties. Different benzodiazepine drugs have different side groups attached to this central structure. The different side groups affect the binding of the molecule to the GABAA receptor and so modulate the pharmacological properties. Many of the pharmacologically active "classical" benzodiazepine drugs contain the 5-phenyl-1H-benzo[e][1,4]diazepin-2(3H)-one substructure. Nonbenzodiazepines also bind to the benzodiazepine binding site on the GABA(A) receptor and possess similar pharmacological properties. While the nonbenzodiazepines are by definition structurally unrelated to the benzodiazepines, both classes of drugs possess a common pharmacophore, which explains their binding to a common receptor site
- 2-keto compounds:
- Chlordiazepoxide, Clorazepate, Diazepam, Flurazepam, Halazepam, Prazepam, and others.
- 3-hydroxy compounds:
- Lorazepam, Lormetazepam,Oxazepam, Temazepam
- 7-nitro compounds:
- Clonazepam, Flunitrazepam, Nimetazepam, Nitrazepam
- Triazolo compounds:
- Adinazolam, Alprazolam, Clonazolam, Estazolam, Triazolam
- Imidazo compounds
- Climazolam, Loprazolam, Midazolam
Images
-
(Purepac) alprazolam pills and prescription bottle
-
Alprazolam solved in propylene glycol
-
.5mg (Qualitest) alprazolam pills
-
Lorazepam (Ativan) pill
Links
http://www.dr-bob.org/tips/bzd.html
History of Etizolam on Tripsit.me
Sources
Madsen U, Bräuner-Osborne H, Greenwood JR, Johansen TN, Krogsgaard-Larsen P, Liljefors T, Nielsen M, Frølund B (2005). "GABA and Glutamate receptor ligands and their therapeutic potential in CNS disorders". In Gad SC. Drug Discovery Handbook. Hoboken, N.J: Wiley-Interscience/J. Wiley. pp. 797–907. ISBN 0-471-21384-5. ^ a b Panico, R.; Powell, W. H.; Richer, J. C., eds. (1993). A Guide to IUPAC Nomenclature of Organic Compounds. IUPAC/Blackwell Science. pp. 40–3. ISBN 0-632-03488-2.; Moss GP (1998). "Nomenclature of fused and bridged fused ring systems (IUPAC Recommendations 1998)". Pure Appl Chem 70 (1): 143–216. doi:10.1351/pac199870010143. Olsen RW, Betz H (2006). "GABA and glycine". In Siegel GJ, Albers RW, Brady S, Price DD (eds.). Basic Neurochemistry: Molecular, Cellular and Medical Aspects (7th ed.). Elsevier. pp. 291–302. ISBN 0-12-088397-X. Shorter E (2005). "Benzodiazepines". A Historical Dictionary of Psychiatry. Oxford University Press. pp. 41–2. ISBN 0-19-517668-5. Zavala F (1997). "Benzodiazepines, anxiety and immunity". Pharmacol Ther 75 (3): 199–216. doi:10.1016/S0163-7258(97)00055-7. PMID 9504140. Narimatsu E, Niiya T, Kawamata M, Namiki A (2006). "[The mechanisms of depression by benzodiazepines, barbiturates and propofol of excitatory synaptic transmissions mediated by adenosine neuromodulation]". Masui (in Japanese) 55 (6): 684–91. PMID 16780077. Juergens, MD, Steven M. "Understanding Benzodiazepines". California Society of Addiction Medicine. Retrieved 25 April 2012. Carlo, Pia; Renata Finollo, Anna Ledda, Giovanni Brambilla (January 1989). "Absence of liver DNA fragmentation in rats treated with high oral doses of 32 benzodiazepine drugs". Fundamental and Applied Toxicology 12 (1): 34–41. doi:10.1016/0272-0590(89)90059-6. PMID 2925017.